Abstract
Background: Thrombosis and thrombocytopenia are both frequent complications in patients with active cancer. Thrombocytopenia can complicate decisions around anticoagulation in cancer associated thrombosis (CAT) but data to guide clinicians is limited. Much of the data on the safety and efficacy of anticoagulation in patients with CAT and thrombocytopenia have been retrospective, single-center, cohort studies. The benefit and risk of direct oral anticoagulants in cancer patients with acute venous thromboembolism (VTE) and thrombocytopenia is incompletely understood. The HOKUSAI VTE Cancer study was a randomized open-label, non-inferiority, phase III trial in CAT that compared dalteparin with edoxaban for at least 6 months. We conducted a post hoc analysis of this trial to investigate the impact of platelet count on bleeding and recurrent thrombosis.
Methods: Patients with platelet counts less than 100,000/µL at specified time points (enrollment, day 31 or day 90) were compared with patients with platelet count > 100,000/µL on all three time points. The primary outcomes for these analyses was major bleeding; secondary outcomes were clinically relevant non-major bleeding (CRNMB), recurrent thrombosis and survival. Cumulative incidences of bleeding and thrombosis outcomes were calculated with death as a competing risk.
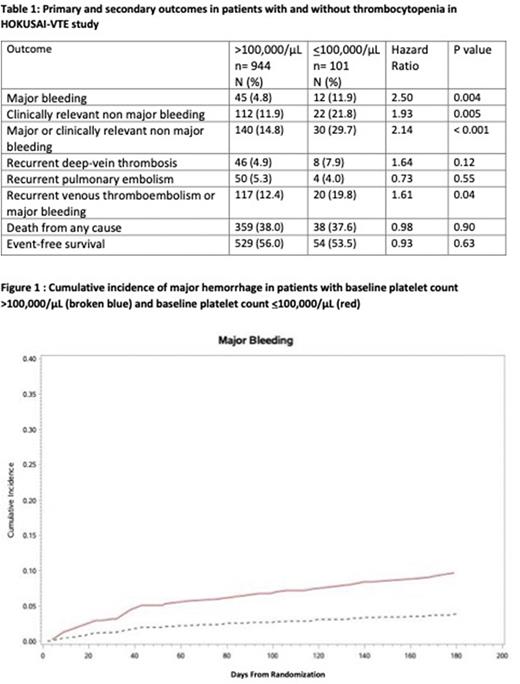
Results: A total of 1045 patients were included in the analyses. , The median age was 65 years and 52% were male. Most common malignancies included colorectal (16%), lung (15%), genitourinary (13%), breast (12%), and hematologic malignancies (11%). Of patients with solid cancers, 53% had metastatic disease and 30% recurrent disease at enrollment. Qualifying thrombotic events included pulmonary embolism for 63% and isolated deep vein thrombosis in 37%. Thrombocytopenia (<100,000/µL) was present in 101 patients (9.6%) of the total cohort. Only 14 patients had a platelet count <50,000/µL at any of the three time points. For the overall trial period the rates of major bleeding were significantly higher in patients with platelet count <100,000/µL (11.9% vs. 4.8%, HR 2.5 P=0.004). (Table 1) Rates of CRNMB were similarly higher in patients with platelet count <100,000/ µL (21.8% vs. 11.9%, HR 1.93, P=0.005), however there was no significant difference in rates of recurrent VTE (11.9% vs. 9.2%, HR 1.28 P=0.42) or overall mortality (37.6% vs. 38%, HR 0.98, P=0.90). Cumulative incidence of major bleeding at 180 days was higher 8.9% (95% CI 3.4-14.5%) in patients with platelet count <100,000/ µL compared to 3.9% (95% CI 2.7-5.2%) in those higher than 100,000/µL. (Figure 1)
Conclusion: In this post hoc analysis of a randomized controlled study in CAT, thrombocytopenia (<100,000/µL) was associated with an increased risk of major bleeding by two-fold. No difference in the risk of recurrent thrombosis was detected. These data highlight the importance of recognizing even mild thrombocytopenia as an important risk factor for bleeding in patients being anticoagulated for CAT.
Disclosures
Shi:Daichi Sankya Pharma Development: Current Employment. Grosso:Daichi Sankya Pharma Development: Current Employment. Duggal:Daichi Sankya Pharma Development: Current Employment. Raskob:North American Thrombosis Forum: Consultancy; ACC Oklahoma: Honoraria; Abbvie Inc: Current equity holder in publicly-traded company; Acadia: Current equity holder in publicly-traded company; Portola Pharmaceuticals: Consultancy; Sangamo Therapeutics Inc: Current equity holder in publicly-traded company; EsperionTherapeutics: Current equity holder in publicly-traded company; Eli Lilly and Company: Consultancy, Current equity holder in publicly-traded company; Canadian Cardiovascular Society: Honoraria; Gilead Sciences Inc: Current equity holder in publicly-traded company; Bristol Mayer Squibb: Consultancy; Biomarin: Current equity holder in publicly-traded company; GlaxoSmithKine, LLC: Current equity holder in publicly-traded company; Incyte Corporation: Current equity holder in publicly-traded company; Teherex: Consultancy; Vaxart: Current equity holder in publicly-traded company; Novartis: Consultancy; Xa Tek: Consultancy; Ziopharm Oncology: Current equity holder in publicly-traded company; NKTR: Current equity holder in publicly-traded company; Pfizer: Consultancy, Current equity holder in publicly-traded company; Altimmune Inc: Current holder of stock options in a privately-held company; Cohbar: Current equity holder in publicly-traded company; Precigen: Current equity holder in publicly-traded company; Daichi Sankya Inc: Consultancy; AMAG Pharmaceuticals: Consultancy; Anthos Therapeutics: Consultancy; Arch Therpeutics: Current equity holder in publicly-traded company; Atea Pharmaceuticals: Current equity holder in publicly-traded company; Bayer Health Care Pharmaceutical: Consultancy; Itreas: Consultancy; Jannsen Global Services, LLC: Consultancy; Japanese Circulation Society: Honoraria; M3 LTD: Honoraria; MD Magazine: Honoraria; Medscape: Consultancy; Merck: Current equity holder in publicly-traded company; Moderna: Current equity holder in publicly-traded company. Zwicker:Quercegen: Research Funding; Sanofi: Consultancy; CSL: Consultancy; Portola: Honoraria; Pfizer/BMS: Honoraria; Incyte: Research Funding; Daiichi: Honoraria; Parexel: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.